Quote from Jacob Christian on August 11, 2023, 7:37 amFor myself and a lot of other wootz smiths out there, it can be quite difficult to find a source of ferro metals. In this post, I will be talking about FeV specifically and a way to make it from 2 separate sources of vanadium.
Method 1: Reduction from Vanadium Pentoxide (V2O5) and Aluminum (Al).
In method one you will obtain pure vanadium metal from a thermite reaction. The V2O5 is a fine Orange powder and can be readily found on eBay. For the Aluminum you will need very fine powder, something like 200 mesh pyrotechnics grade.
Both the reactions involving V2O5+Al and Fe2O3+Al are exothermic reactions, meaning they release heat energy during the reaction. However, there are differences in terms of the products formed and the thermodynamics of the reactions.
- V2O5 + Al: The reaction between vanadium pentoxide (V2O5) and aluminum (Al) produces vanadium metal (V) and aluminum oxide (Al2O3) as products. This reaction is commonly used for the reduction of vanadium pentoxide in metallurgical processes.Balanced Reaction: V2O5 + 5 Al → 2 V + 5 Al2O3
In this reaction, vanadium pentoxide is reduced to vanadium metal, and aluminum is oxidized to aluminum oxide. The heat released is due to the difference in energy between the reactants and products. The reaction is highly exothermic and can result in a rapid release of heat, which makes it useful in various applications such as welding and thermite reactions.
- Fe2O3 + Al: The reaction between iron oxide (Fe2O3) and aluminum (Al) produces iron (Fe) and aluminum oxide (Al2O3) as products. This reaction is known as a type of thermite reaction and is used for various purposes, including metal joining and cutting applications.Balanced Reaction: 2 Fe2O3 + 6 Al → 4 Fe + 6 Al2O3
Similar to the V2O5+Al reaction, this reaction is exothermic and releases a significant amount of heat. The energy released comes from the difference in energy between the reactants and products.
In both reactions, the exothermic nature of the reactions is a result of the strong tendency of oxygen to transfer from the metal oxides (V2O5 and Fe2O3) to aluminum, forming the respective metals (V and Fe) and aluminum oxide (Al2O3).
Method 2: Pure Vanadium Crystals
This method is pretty self explanatory compared to the one above. I have not done the math to break down which is cheaper. I do know that the thermite reaction is wayyyyyy cooler though.
High Temperature Alloying
- Alloying: Mix the obtained vanadium metal with electrolytic iron in the desired proportions to achieve a 50% FeV composition (half iron, half vanadium). The alloying process involves melting the materials together at high temperatures to ensure thorough mixing and homogenization of the components. This can be done in a furnace or other appropriate equipment.
- In my specific case, I will be using a small alumina crucible "boat". This is a small 99% alumina crucible that withstands temperature of 1800°C. While Vanadium melts at 1910°C this will still be ok because we do not need to heat the whole crucible, just the vanadium directly.
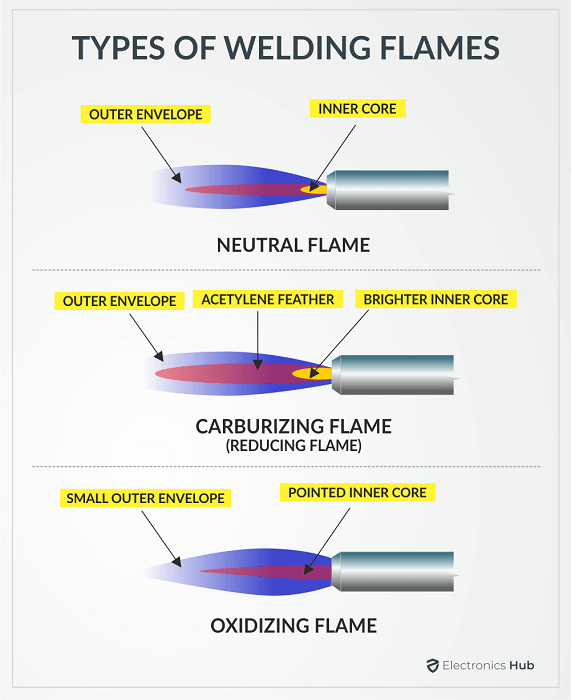
- To melt the ingredients I will be using an oxyacetylene torch which reaches temperatures of 3,320 °C at the tip of the flame. NOTE: You will want to make sure that you are using a carburizing flame on your torch so that you do not oxidize any of the iron. See the picture below for reference.
- When loading the crucible boat, make sure the evenly distribute both the electrolytic iron and vanadium crystals to aid in creating a homogenous alloy.
- First heat up your electrolytic iron to molten temps and then start melting the vanadium crystals using the tip of the oxyacetylene flame. Once everything is melted you can add a couple of pieces of charcoal to the top to help keep that oxidizing atmosphere or leave the oxyacetylene flame running just above the crucible until the contents are solid.
For myself and a lot of other wootz smiths out there, it can be quite difficult to find a source of ferro metals. In this post, I will be talking about FeV specifically and a way to make it from 2 separate sources of vanadium.
Method 1: Reduction from Vanadium Pentoxide (V2O5) and Aluminum (Al).
In method one you will obtain pure vanadium metal from a thermite reaction. The V2O5 is a fine Orange powder and can be readily found on eBay. For the Aluminum you will need very fine powder, something like 200 mesh pyrotechnics grade.
Both the reactions involving V2O5+Al and Fe2O3+Al are exothermic reactions, meaning they release heat energy during the reaction. However, there are differences in terms of the products formed and the thermodynamics of the reactions.
- V2O5 + Al: The reaction between vanadium pentoxide (V2O5) and aluminum (Al) produces vanadium metal (V) and aluminum oxide (Al2O3) as products. This reaction is commonly used for the reduction of vanadium pentoxide in metallurgical processes.Balanced Reaction: V2O5 + 5 Al → 2 V + 5 Al2O3
In this reaction, vanadium pentoxide is reduced to vanadium metal, and aluminum is oxidized to aluminum oxide. The heat released is due to the difference in energy between the reactants and products. The reaction is highly exothermic and can result in a rapid release of heat, which makes it useful in various applications such as welding and thermite reactions.
- Fe2O3 + Al: The reaction between iron oxide (Fe2O3) and aluminum (Al) produces iron (Fe) and aluminum oxide (Al2O3) as products. This reaction is known as a type of thermite reaction and is used for various purposes, including metal joining and cutting applications.Balanced Reaction: 2 Fe2O3 + 6 Al → 4 Fe + 6 Al2O3
Similar to the V2O5+Al reaction, this reaction is exothermic and releases a significant amount of heat. The energy released comes from the difference in energy between the reactants and products.
In both reactions, the exothermic nature of the reactions is a result of the strong tendency of oxygen to transfer from the metal oxides (V2O5 and Fe2O3) to aluminum, forming the respective metals (V and Fe) and aluminum oxide (Al2O3).
Method 2: Pure Vanadium Crystals
This method is pretty self explanatory compared to the one above. I have not done the math to break down which is cheaper. I do know that the thermite reaction is wayyyyyy cooler though.
High Temperature Alloying
- Alloying: Mix the obtained vanadium metal with electrolytic iron in the desired proportions to achieve a 50% FeV composition (half iron, half vanadium). The alloying process involves melting the materials together at high temperatures to ensure thorough mixing and homogenization of the components. This can be done in a furnace or other appropriate equipment.
- In my specific case, I will be using a small alumina crucible "boat". This is a small 99% alumina crucible that withstands temperature of 1800°C. While Vanadium melts at 1910°C this will still be ok because we do not need to heat the whole crucible, just the vanadium directly.
- To melt the ingredients I will be using an oxyacetylene torch which reaches temperatures of 3,320 °C at the tip of the flame. NOTE: You will want to make sure that you are using a carburizing flame on your torch so that you do not oxidize any of the iron. See the picture below for reference.
- When loading the crucible boat, make sure the evenly distribute both the electrolytic iron and vanadium crystals to aid in creating a homogenous alloy.
- First heat up your electrolytic iron to molten temps and then start melting the vanadium crystals using the tip of the oxyacetylene flame. Once everything is melted you can add a couple of pieces of charcoal to the top to help keep that oxidizing atmosphere or leave the oxyacetylene flame running just above the crucible until the contents are solid.
Uploaded files:
Quote from Raggedyman on August 5, 2024, 12:16 pmI have to ask, why the particular desire for a pure ferro-vanadium source?
Is it to recreate historical authenticity in the steel as far as possible?
Relatively easy to get and cheap steels like 115CrV3 have little enough of anything else in them that I expect it would suffice at around a 1 to 10 ratio.
Am I missing something?
I have to ask, why the particular desire for a pure ferro-vanadium source?
Is it to recreate historical authenticity in the steel as far as possible?
Relatively easy to get and cheap steels like 115CrV3 have little enough of anything else in them that I expect it would suffice at around a 1 to 10 ratio.
Am I missing something?